Story highlights
A study on the safety and effectiveness of cord blood in children with autism yielded promising results
"She got better, and we're just thankful for that -- whether it be the stem cells or not," one family says
Gracie Gregory smiles beneath her brilliant blue eyes. She’s sitting on her mother’s lap, next to her older sister, Ryleigh, who boasts about Gracie being “very sweet and kind.”
It wasn’t always so. Just a couple years ago, Ryleigh, 11, was scared of her sister when she’d throw tantrums and screaming fits.
“She would’ve fought and kicked,” Ryleigh says, noting that it wouldn’t have been possible to sit like this next to Gracie.
Why was she scared of her sister?
“Because of the kicking.”
Gracie, 7, interrupts: “I don’t even remember it.”
“We do,” says her mother, Gina Gregory.
Gracie has autism, a condition that affected nearly every aspect of her family’s life after she was diagnosed at 2. But a new study is offering hope for the Gregorys and families like them.
Gracie was one of 25 children who took part in the first-of-its-kind study at Duke University in Durham, North Carolina. The goal: to see whether a transfusion of their own umbilical cord blood containing rare stem cells could help treat their autism.
The results were impressive: More than two-thirds of the children showed reported improvements. A larger second trial is underway, one its researchers hope will lead to long-term treatment for children with autism.
Skeptics say there are too many unanswered questions to get excited. Even Duke researchers acknowledge as much. The initial trial, published Wednesday in the journal Stem Cells Translational Medicine, was a safety study, not a controlled, double-blind study with definitive proof of positive results. This study was open-label, meaning everyone – the doctors and the families – knew that the therapy was being administered.
But for the Gregorys, the change in their daughter has been monumental.

Gone are the days of Gracie throwing fits in long lines at Disney World or during dinner at restaurants. When a tantrum intruded on family outings, her mom and dad wished they had T-shirts that said “My kid has autism” to ward off judgmental stares.
During autism therapy sessions, Gracie would kick, scream, spit and hit at her occupational therapist. “It was horrible to try to get her to sit there,” her mother says.
Even just brushing her teeth or combing her hair could set her off.
Gracie, then 5, was on the mild to moderate autism scale, but her parents say the disorder consumed about 75% of their daily routine. After her participation in the study, that figure has been reduced to a mere 10%.
On a scale of 1 to 10, they rate her improvement around an 8 or 9; it’s been that dramatic. She’s even begun attending a “regular” school and thriving there, something her parents never thought possible. She’d been in various specialized school programs, and nothing was the proper fit.
Are Gracie’s changes a result of the cord blood transfusion stimulating her brain? Or did her brain just mature as she got older? Could it be that her parents were subconsciously determined to magnify her improvements, given all their family had been through?
Those are questions the Gregorys still ask. But they do know that their daughter’s transformation appeared to begin about six months after her transfusion in January 2015 and has continued ever since.
Her father’s favorite adjustment is her newfound affection. Instead of shunning hugs, she now welcomes an embrace.
“We will say we don’t think it’s cured her. You still see some of the small idiosyncrasies that she does have,” says her father, Wade Gregory. “But again, I think it’s supercharged her learning curve. It’s pushed her to do things she normally wouldn’t do.”
Her mother adds, “She got better, and we’re just thankful for that – whether it be the stem cells or not. We’re just thankful for what changes have happened.”

Billions of cells
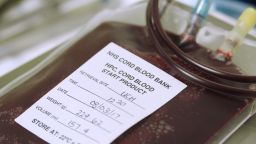
Dr. Joanne Kurtzberg shows off a freezer deep inside the bowels of the Carolinas Cord Blood Bank at Duke University Medical Center. Known as a thermogenesis freezer, it stores up to 3,640 units of cord blood – left over from babies’ umbilical cords and placenta – at minus 196 degrees Celsius.
Each unit is designated by labels with specially designed adhesive to withstand extremely cold temperatures for decades. There are 14 cord blood freezers in all.
It is the cord blood in those freezers – stored or donated by parents in case a serious illness develops – that’s at the cutting edge of this research.
Kurtzberg, who heads the Robertson Clinical and Translational Cell Therapy Program, has teamed up with Dr. Geraldine Dawson, director of the Duke Center for Autism and Brain Development.
Both saw a great need for medical advances to help treat children with autism. An estimated one in every 68 children in America has some form of autism spectrum disorder, according to the Centers for Disease Control and Prevention.
About 30% never learn to speak, and many children even with early behavioral interventions still struggle to adapt. There also are no FDA-approved medications that improve the core symptoms of autism.
“I was very interested in collaborating with people here at Duke who could offer medical approaches that could enhance neuroplasticity, or the brain’s ability to respond to treatment,” Dawson says.
That’s where Kurtzberg comes in. Over the past two decades, she had seen children with inherited metabolic disorders be treated with cord blood after receiving high doses of chemotherapy.
“We’ve been able to show that with some of these diseases, a cord transplant rescues them from death and also improves their neurologic outcome,” she says.
She began wondering: Could cord blood help other children?
About a decade ago, her laboratory began clinical tests of children with cerebral palsy whose parents had banked their cord blood. Again, they saw positive results. And in some of those children who had autistic tendencies, they saw autistic symptoms improve. Another spark went off: What if they tested cord blood specifically for autism?

The safety trial began a little over a year and a half ago. Not only did it find cord blood to be safe, but 70% of the 25 children, age 2 to 6, had behavioral improvements as described by their parents and tracked by the Duke researchers. The research is largely funded by a $40 million donation from the Marcus Foundation, a nonprofit created by Home Depot co-founder Bernie Marcus.
The children traveled to Duke three times over the course of a year. They underwent a series of evaluations such as autism assessments, MRIs and EEGs to track their brain activity. On the first trip, the children received the cord blood infusion along with the intense evaluations. Each child received 1 billion to 2 billion cells, given through an IV in their arms or legs. At six months and then a year later, the children returned for more tests and observations.
“Some children, who were not speaking very much, had big increases in their vocabulary and their functional speech,” Kurtzberg says. “Many children were able to attend to play and have meaningful communication in a way that they weren’t before. Some children had less repetitive behaviors than they did when they came onto the study.”
Adds Dawson, “The study was very encouraging. We did see positive results. However, it did not have a comparison group, which is very important in establishing whether a treatment is actually effective.”
Both researchers can’t stress that enough: that although they’re cautiously optimistic about the results, they want the science to play out. They are now in the midst of the definitive trial on whether cord blood can treat autism – a double-blind, placebo-controlled trial involving 165 autistic children, ranging in age from 2 to 8. The FDA has oversight of the study.
During the phase II study, the children on their first visit receive a cord blood infusion – either their own or from a donor – or they get a placebo. They also undergo a battery of assessment tests and brain monitoring.
On their second visit six months later, the children receive a second infusion with whatever preparation they did not receive the first time and undergo more evaluations. The order of the infusions is not known. Researchers will monitor them for the next year for any sign of behavioral improvements.
It’s known as a crossover trial, in which each subject gets the treatment and the placebo but in a different order. Researchers say it would have been nearly impossible to find participants if parents knew that their children might not receive an infusion.
How groundbreaking would it be if the trial shows similar results to the safety study?
“If we can show that receiving an infusion of cord blood is more effective for improving social behavior than the placebo,” Dawson says, “then this will be game-changing.”
Kurtzberg adds, “We’ll be extraordinarily encouraged if the second trial shows that the cells benefit children when the placebo does not. We will consider that a breakthrough.”

Both researchers were shaped early in life by the struggles families face raising autistic children. As a teen, Dawson babysat twins with autism who lived across the street. “It was just an inspiration to devote my career to improving the lives of people with autism,” she says.
Kurtzberg was similarly affected. When she was a junior in college, she would visit a girl with severe autism and play with her as a means of behavioral intervention. “The family still writes to me,” she says.
It is for this reason – their longtime devotion to families raising children with autism – that both issue a heap of caution. Although they’re excited about the results of the first study, Kurtzberg says, “we don’t want to mislead people and claim it’s working before we have definitive proof.”
Adds Dawson, “It’s important for parents who might hear about cord blood as a potential treatment for autism to know that we are working very hard to know the answer to that question. We aren’t there yet.”
Cautious optimism
Kurtzberg has a hypothesis about what may be happening: that certain immune cells within the cord blood are crossing the blood-brain barrier and altering brain connectivity while also suppressing inflammation, which may exist with autism.
“I feel more confident now because of our (cerebral palsy) study, which preceded this study and does show benefits,” Kurtzberg says.
Dr. Arnold R. Kriegstein, director of the stem cell center at the University of California, San Francisco, says that he hopes there will be breakthrough treatment for children with autism but that much more needs to be known before this will become a reality.
“One has to be very careful when interpreting results that haven’t come from properly controlled, double-blind studies,” he said. “All I can say is that it would be wonderful if this treatment was effective, but one has to be very cautious before reaching any conclusions.”
Even without a placebo effect, he says, many factors could have resulted in an improved outcome in the first study: The growing children could have acquired skills simply through maturation, possibly enhanced by occupational therapy, and their parents may have clung to positive gains, creating a biased outcome.
Thomas Frazier II, chief scientific officer of the advocacy group Autism Speaks, said the results of the initial study were encouraging but that more work needs to be done before the public gets excited. “It’s too early to get hopeful. Too early to change behavior,” he said. “I hope people don’t go out and spend money on banking cord blood as a result [of this trial].”
Kriegstein of UCSF also wonders whether cord blood is really stimulating cells in the brain and creating new connections. “There are so many unanswered questions about what might be going on here, it becomes very difficult to evaluate the proposed mechanism,” he said.
“The question remains: How do these cells injected intravenously wind up in the brain, how do they target the appropriate brain regions, and what are they doing that could improve brain function?”

‘I forgot how bad it was’
An 8-year-old boy with autism sits at a table in a room within Duke’s Center for Autism and Brain Development. Clinical research specialist Michelle Green watches from behind a two-way mirror. Two cameras in the room feed computer monitors, allowing her to further analyze his behavior.
Dr. Lauren Franz, a clinician, works with the boy in the room.
“What kind of things make you feel threatened or anxious?” she asks.
“Like when I’m done with a test,” the boy says.
“How does it feel when you’re frightened or anxious? How does that feel?”
“Like pretty weird,” he says.
The boy is participating in the second trial, and he’s returned for his six-month assessment and second infusion. Researchers don’t know which infusion he received first: the cord blood or the placebo.
But they track, record and monitor the slightest of details. Although it might seem like an innocuous conversation, researchers will compare the results with those of his first visit and any follow-ups. Was he able to sit still at the table before? Could he articulate his thoughts? Did he talk before the study? Has he improved?
Join the conversation
At the Gregorys’ home in Florida, Gracie’s parents remember when she went through those same tests. The best investment they ever made, they say, was the $2,000 spent on banking her cord blood. At the time, it was just a precaution; her autism diagnosis didn’t come until three months after her second birthday.
They know the desperation of families raising a child with autism – of longing for their daughter to have a shot of normalcy in life. “You can’t quantify it. You can’t measure it. You want to see your child succeed,” her father says.
Mom and Dad recently watched old home videos, of Gracie singing inaudibly, of her covering her ears when “Happy Birthday” was sung for her third birthday, of showing no emotion on Christmas when she was 2. “I forgot how bad it was,” her mother says.
They hope the current study leads to similar successes – and results in breakthrough treatment for autistic children everywhere.